Key Features of Corpex-DC
Thursday, November 30, 2023
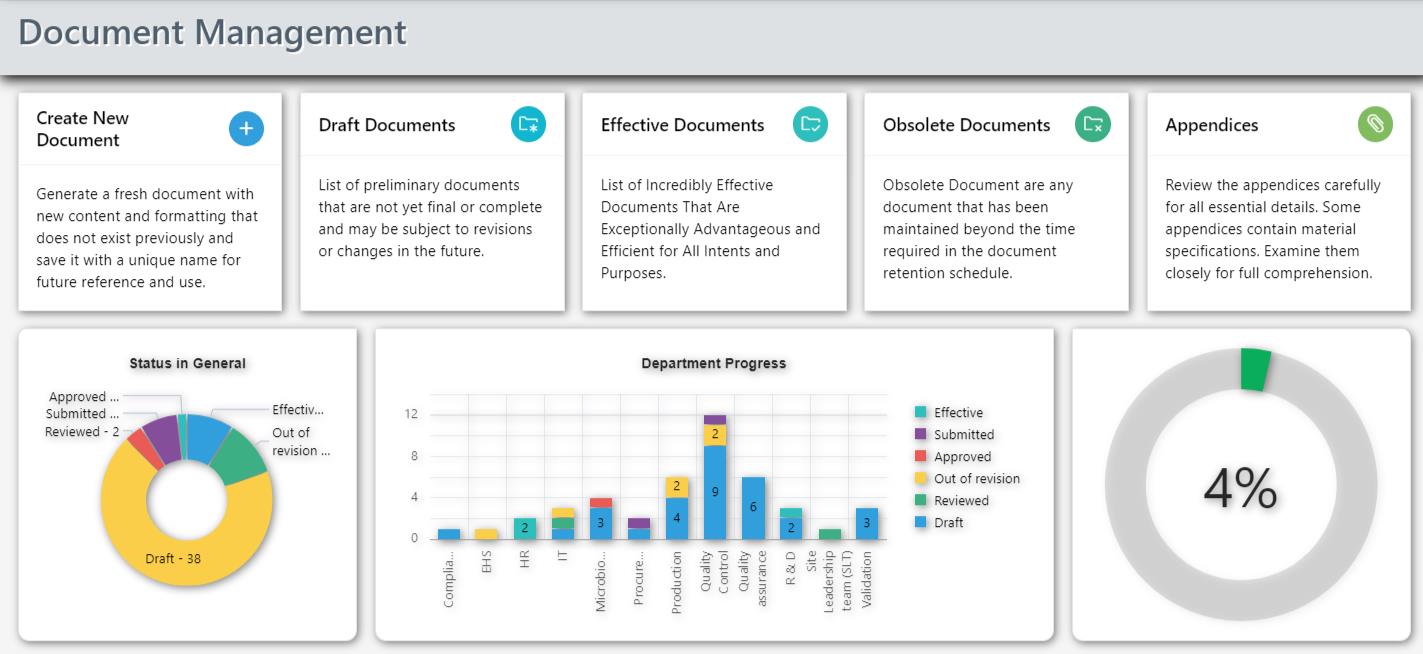
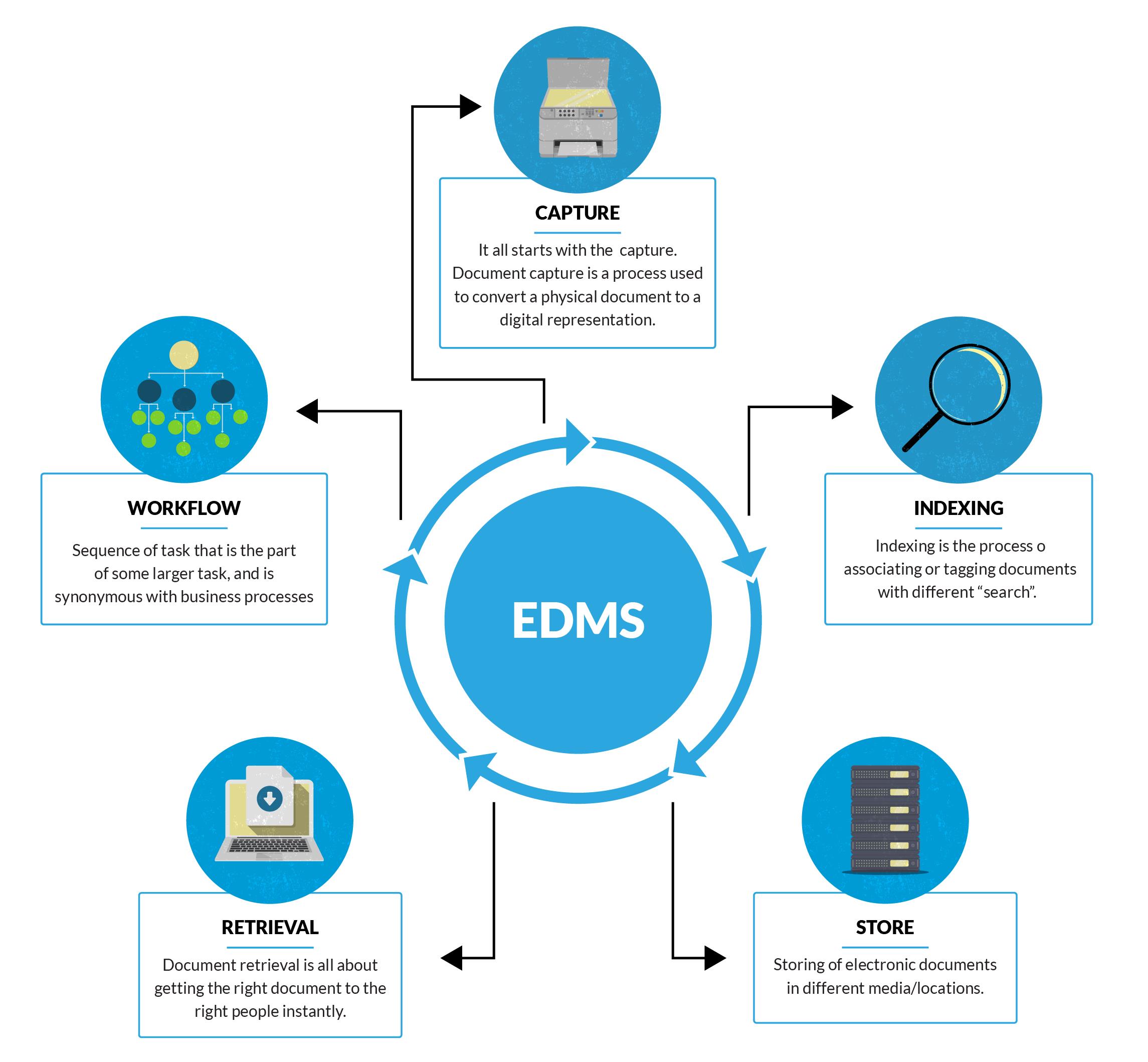
Centralized Repository:
Corpex-QMS provides a centralized repository for all quality-related documents, eliminating the challenges associated with scattered and disparate document storage. This centralized approach ensures that users can easily locate, access, and collaborate on documents, fostering a collaborative and organized work environment.
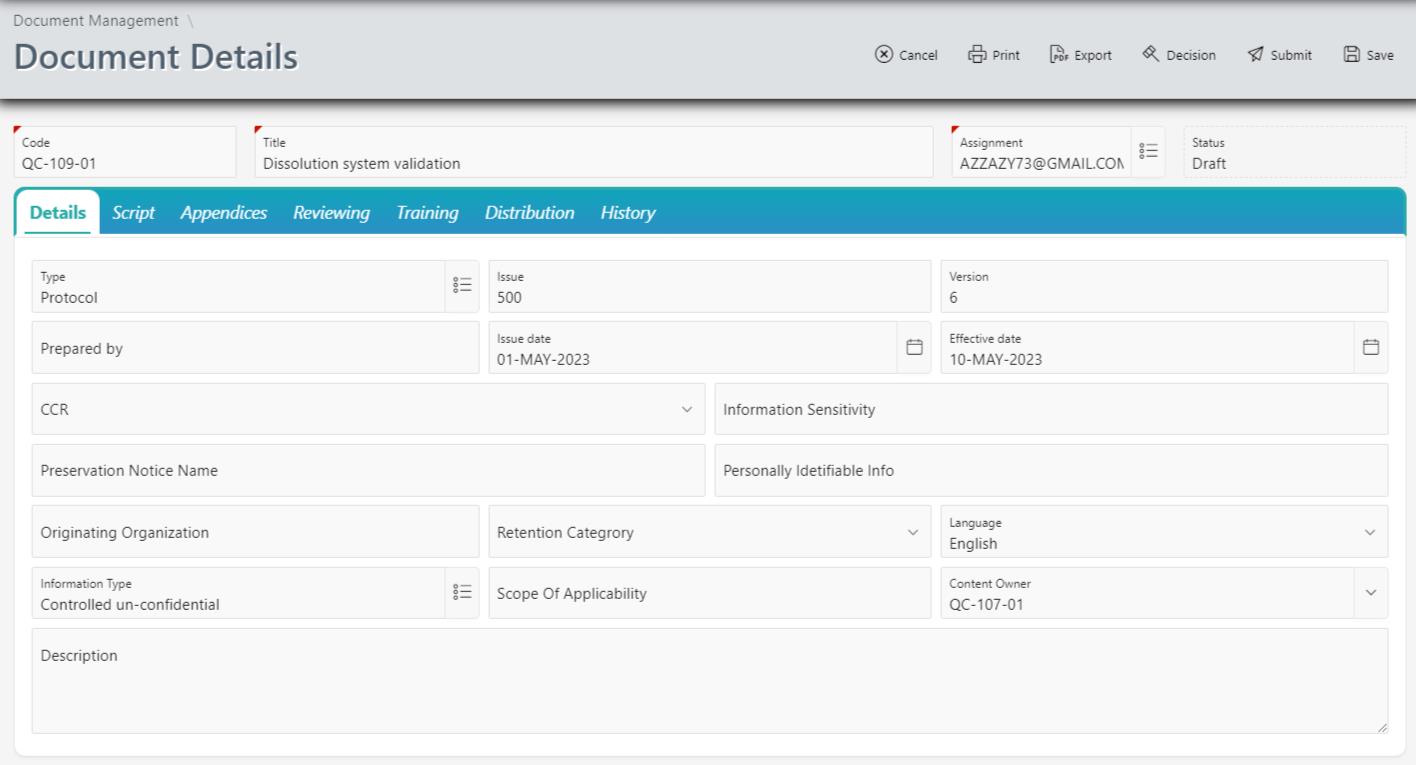
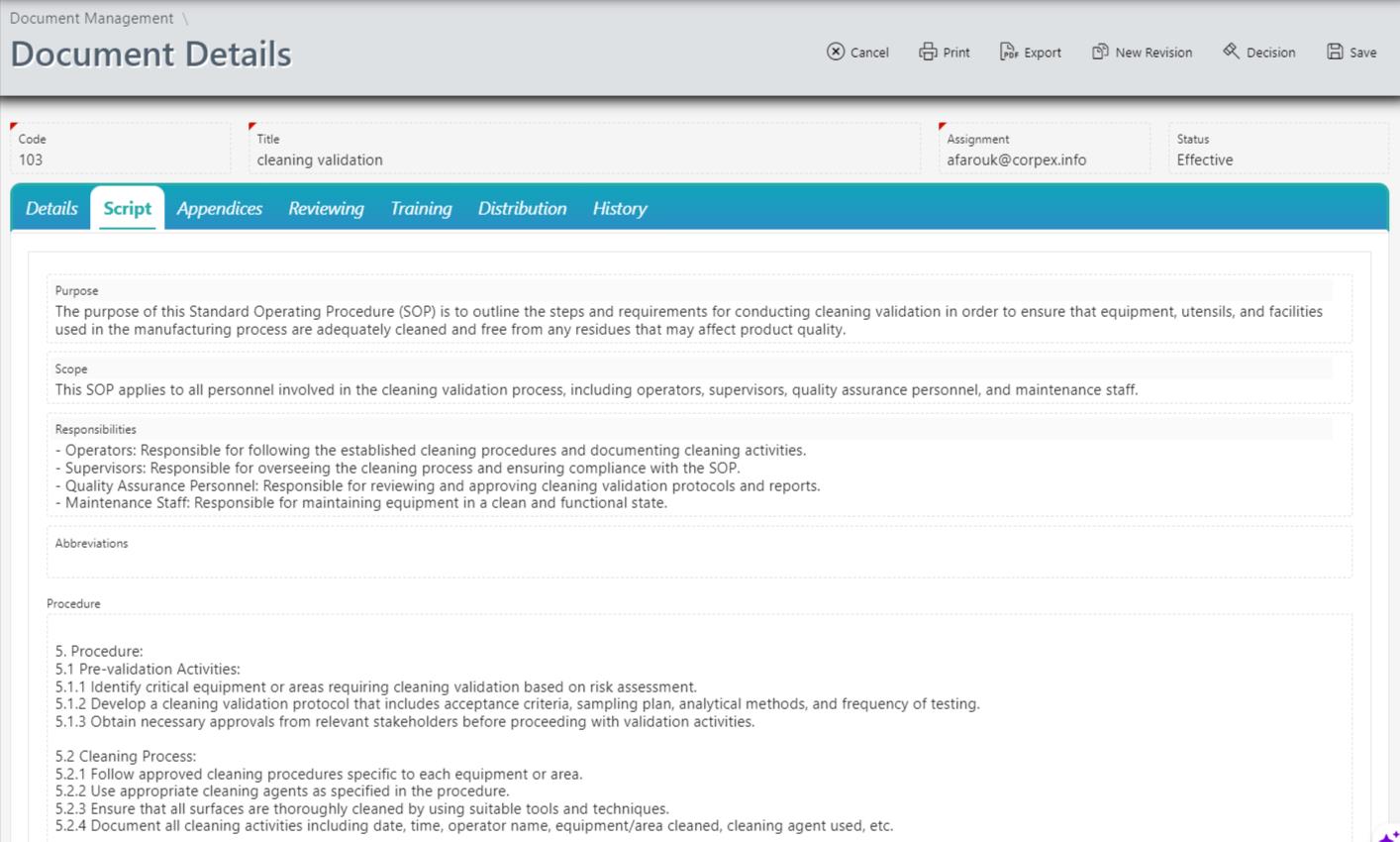
Version Control:
Version control is a critical aspect of document management, and Corpex-QMS excels in this regard. The Document Management Module enables organizations to maintain a complete version history of each document, tracking changes made over time. This ensures that users always access the latest, approved version of a document, minimizing the risk of errors and ensuring compliance.
Access Control and Security:
Security is paramount when managing sensitive documents. Corpex-QMS offers robust access control mechanisms, allowing organizations to define user roles and permissions. This ensures that only authorized personnel can access, edit, or approve documents, safeguarding confidential information and maintaining the integrity of the QMS.
Workflow Automation:
The Document Management Module in Corpex-QMS streamlines document approval processes through workflow automation. Organizations can define and customize workflows to match their specific document approval processes, reducing manual interventions, and ensuring a seamless and efficient approval cycle.
Electronic Signatures:
In compliance-driven industries, electronic signatures are indispensable. Corpex-QMS supports electronic signatures, providing a secure and legally recognized method for document approvals. This feature enhances the efficiency of document workflows while maintaining compliance with regulatory requirements.
Audit Trail:
Transparency is a cornerstone of effective quality management. Corpex-QMS Document Management Module includes an audit trail feature, allowing organizations to track and monitor all document-related activities. This feature is invaluable during audits, providing a comprehensive record of document changes, approvals, and user interactions.
Conclusion
In the ever-evolving landscape of quality management, the Document Management Module in Corpex-QMS emerges as a cornerstone for organizations seeking to establish and maintain effective quality processes. By offering a centralized repository, robust version control, stringent access controls, workflow automation, electronic signatures, and an audit trail, Corpex-QMS empowers organizations to streamline their document management processes, ensuring compliance, enhancing collaboration, and driving continuous improvement initiatives. As organizations strive to meet and exceed quality standards, the Document Management Module in Corpex-QMS stands as a testament to the commitment to excellence in quality management.