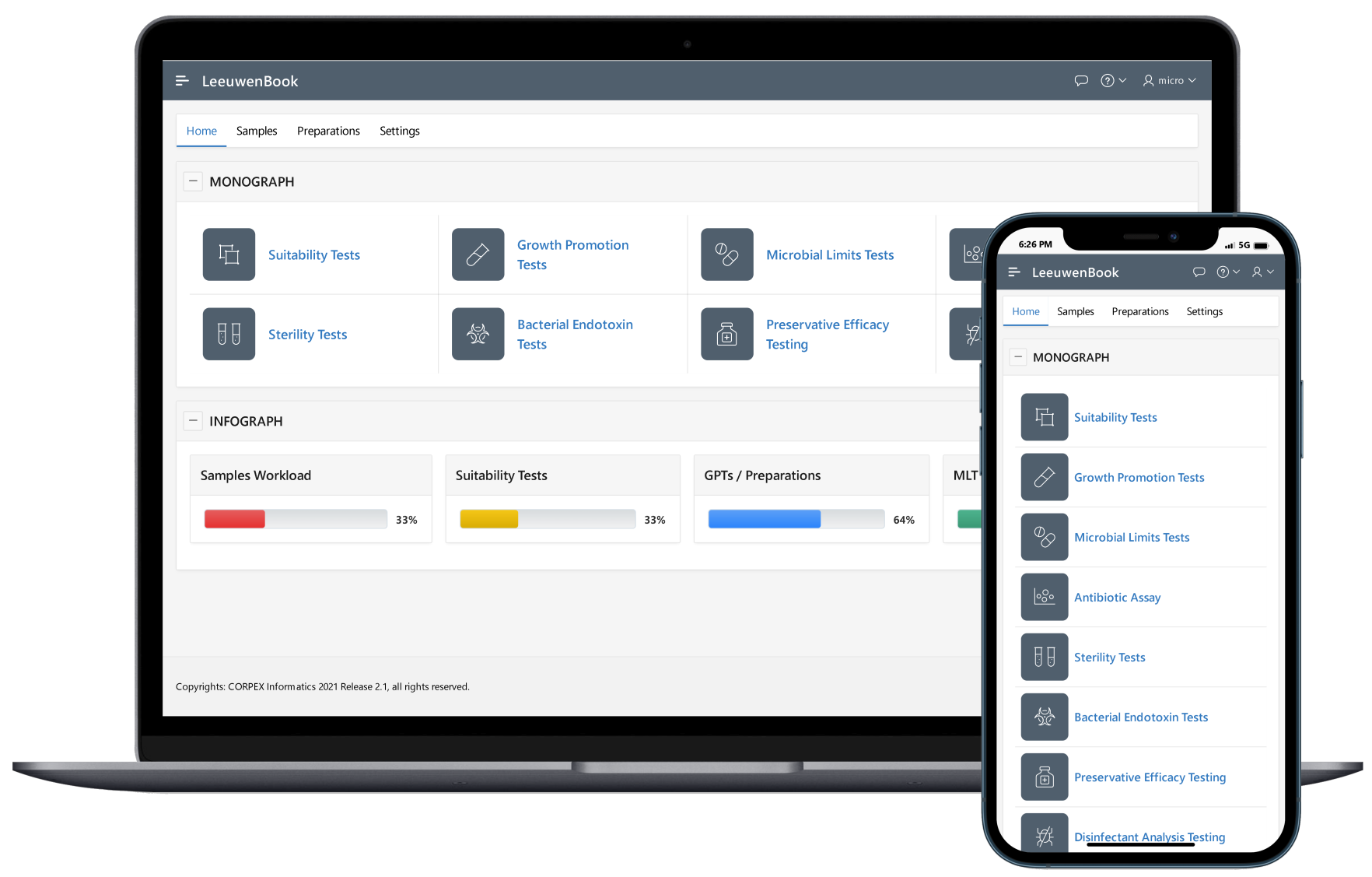
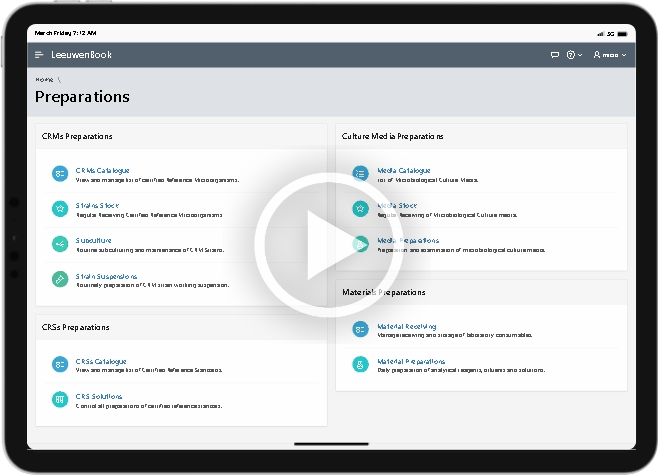







Microbial culture media preparation is a routine task in the regular detection, count and monitoring of microbial flora in microbiological testing. Microbial culture media preparation is the process of mixing nutrients, agents for buffering and maintaining the osmotic balance, as well as selective inhibitors or indicators to create an agar or broth that supports the growth and the differentiation of microorganisms. Leewuenbook provides recording of all pertaining information for culture media, subculture of Certified Reference Microorganisms (CRMs) and other preparations that support integration of laboratory preparations.
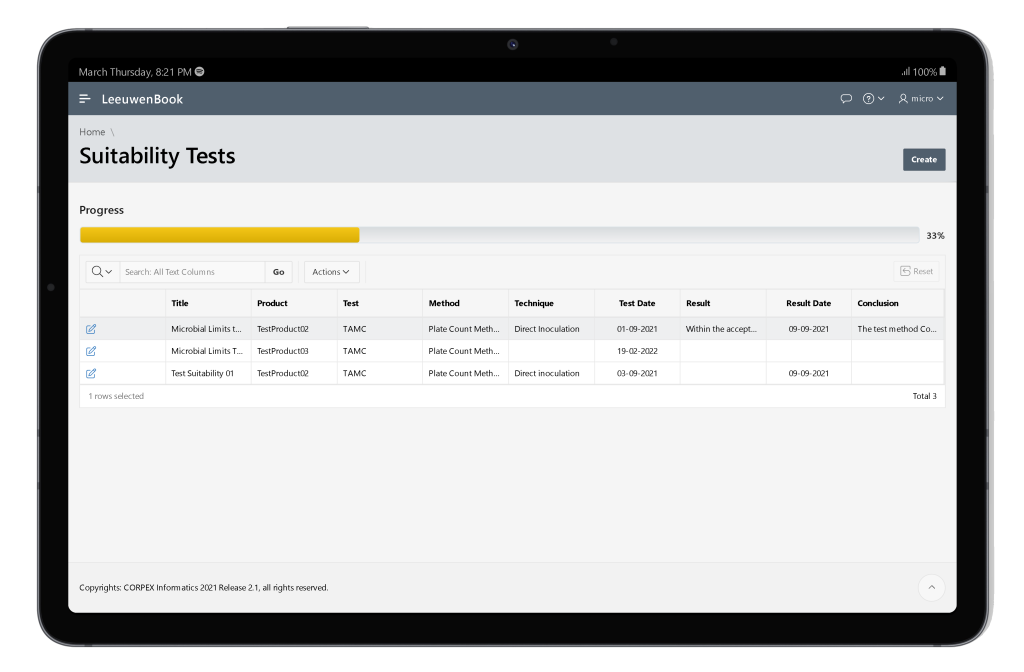
The goal of a microbiological method suitability test is to demonstrate that any residual antimicrobial properties of the product or the recovery method have been neutralized using the challenge microorganisms as a kind of biological indicator of neutralization. The method of assessing if neutralization has been successful varies depending on the method employed. The ability of the test to detect microorganisms in the presence of product to be tested must be established (USP 41). Therefore, Leeuwenbook provides integration of pertaining informations of varies suitability methods.
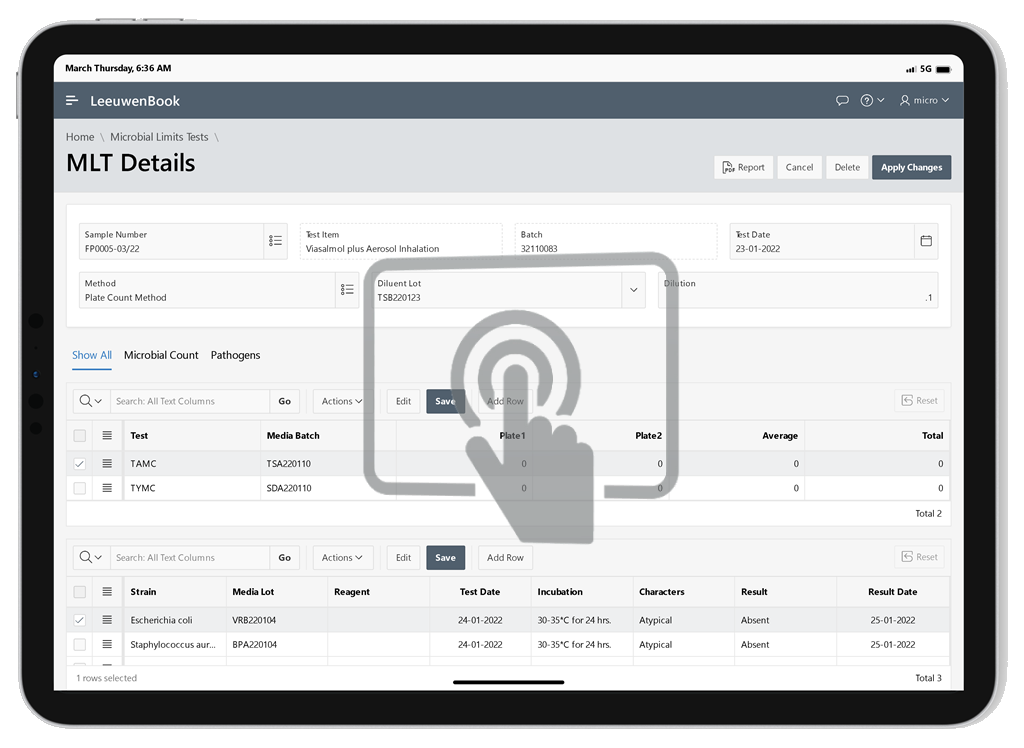
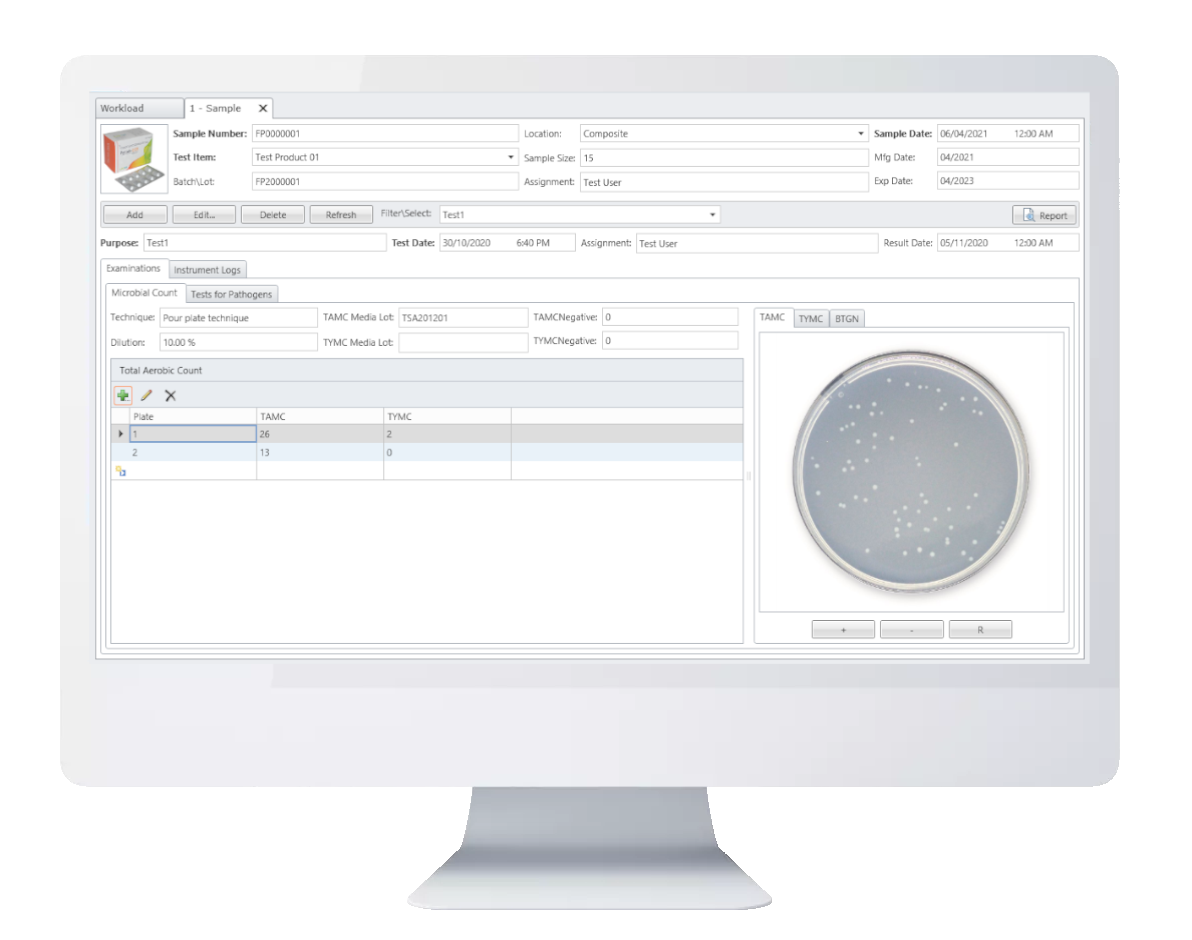
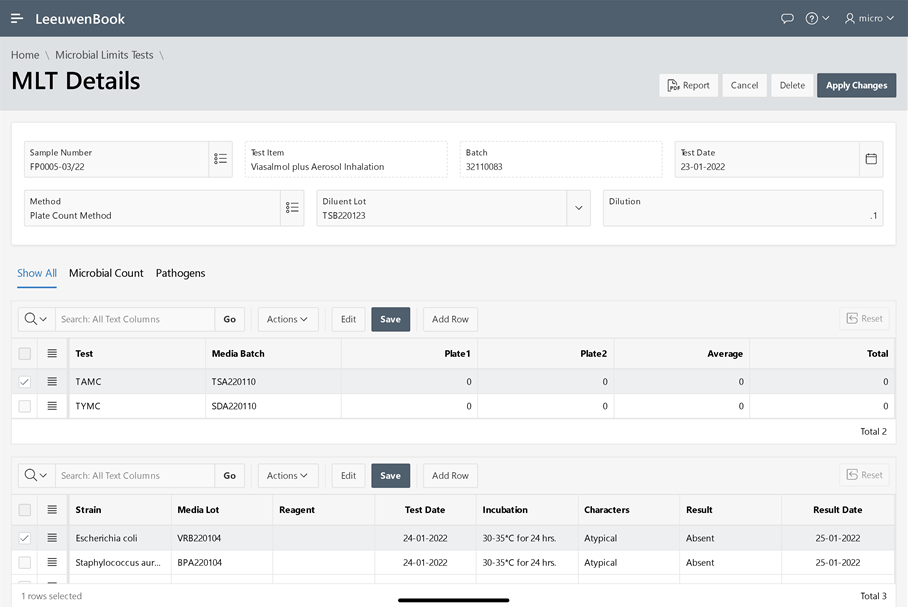
The microbial limit test (MLT) is performed to assess how many and which of certain viable microorganisms are present in non-sterile pharmaceutical, healthcare or cosmetics manufacturing samples that range from raw materials to finished products. USP tests allow quantitative enumeration of mesophilic bacteria and fungi that may grow under aerobic conditions. The tests are designed primarily to determine whether a substance or preparation complies with an established specification for microbiological quality. Leeuwenbook provides integration of pertaining informations of microbial limits tests.
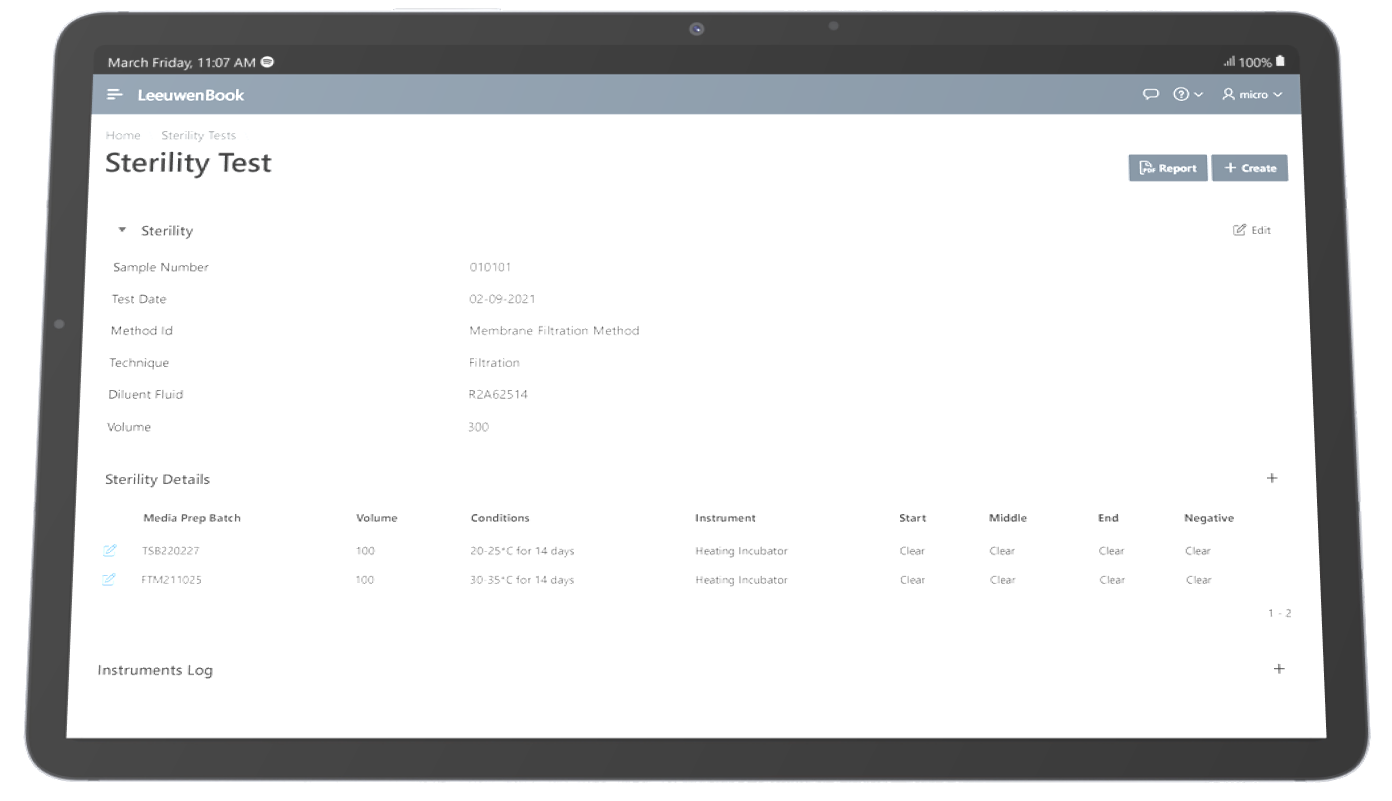
Sterility testing is a GMP microbiology testing requirement used to confirm sterile products do not contain viable microorganisms before release and patient administration. Sterility testing methods must be as accurate as possible, due to their importance for medical devices, pharmaceutical products, and formulations, tissue materials, and other products that claim to be sterile or free from viable microorganisms. Compendial methods for the sterility testing of pharmaceutical products requires samples to be cultured in two separate media. Two different types of culture media are used in sterility testing to promote the growth of residual anaerobes, as well as aerobes and fungi. Fluid thioglycolate medium (FTM) is typically used to culture anaerobic and some aerobic bacteria, while soybean casein digest medium (SCDM) is typically used to culture fungi and aerobic bacteria. Samples are incubated for 14 days at 32.5°C and 22.5°C respectively, prior to examination. Any turbidity in the culture media may indicate growth and must be investigated. There are two recommended methods of sterility testing for pharmaceuticals: membrane filtration and direct inoculation.

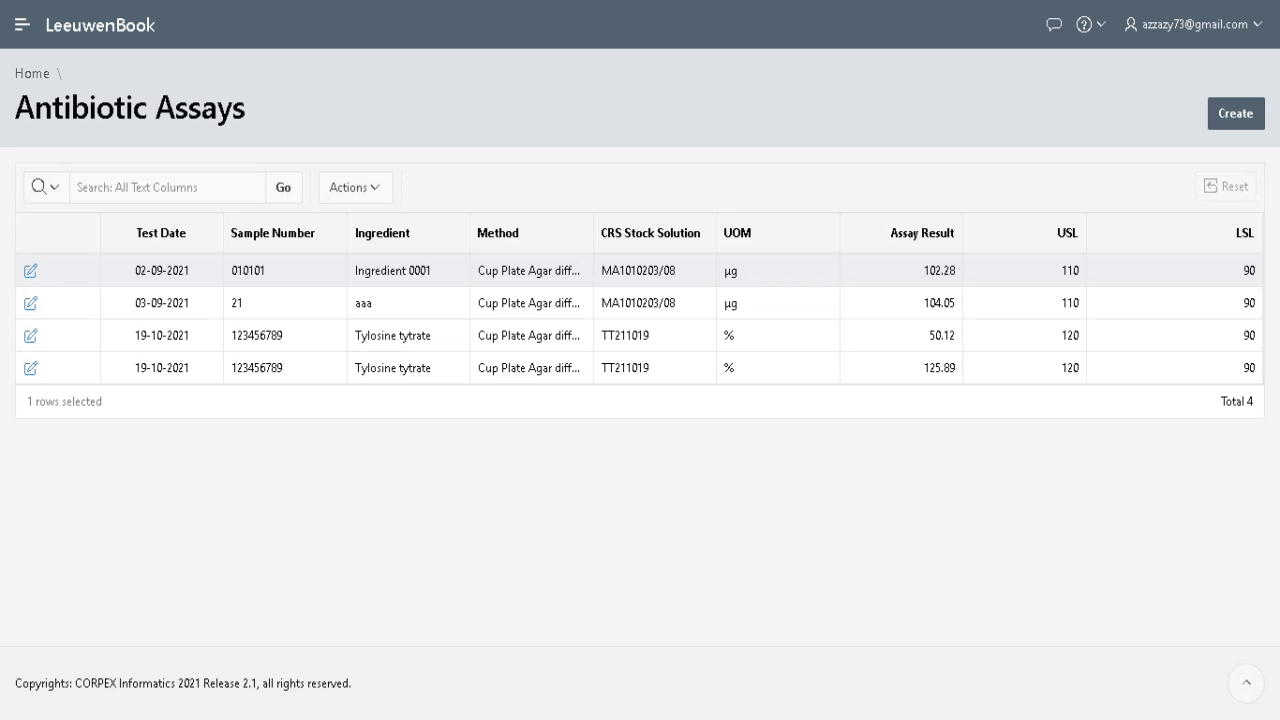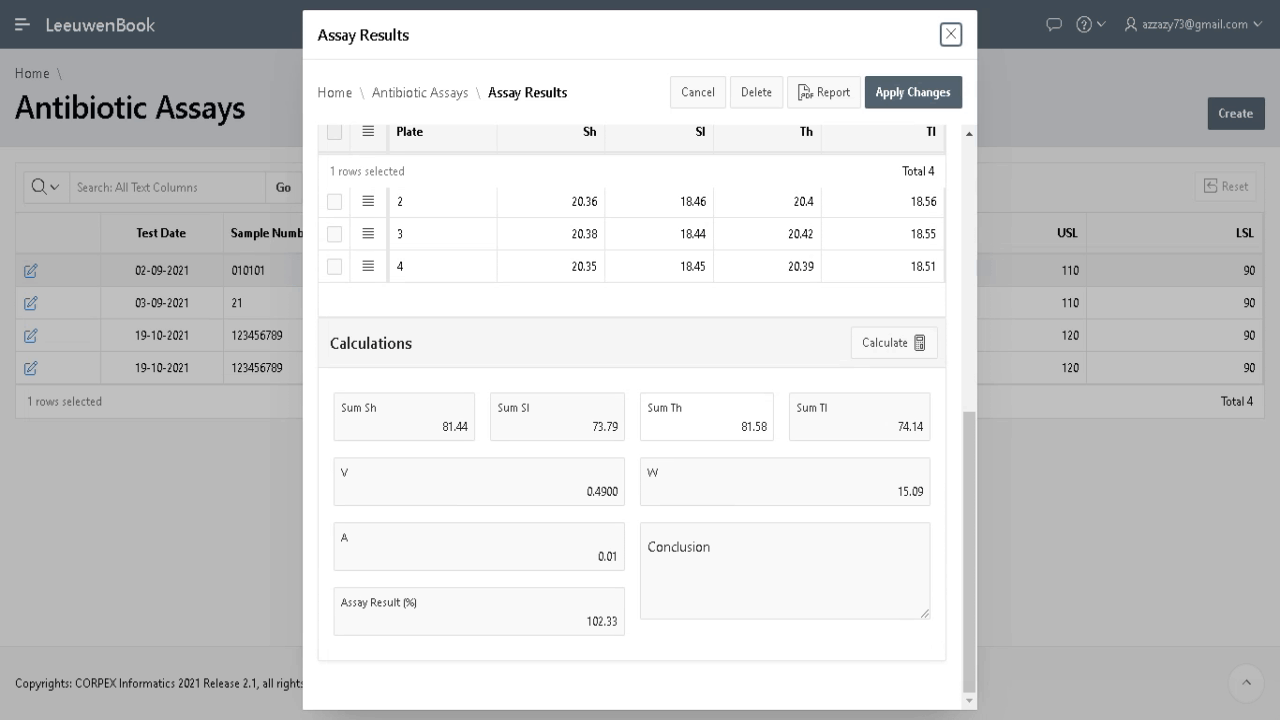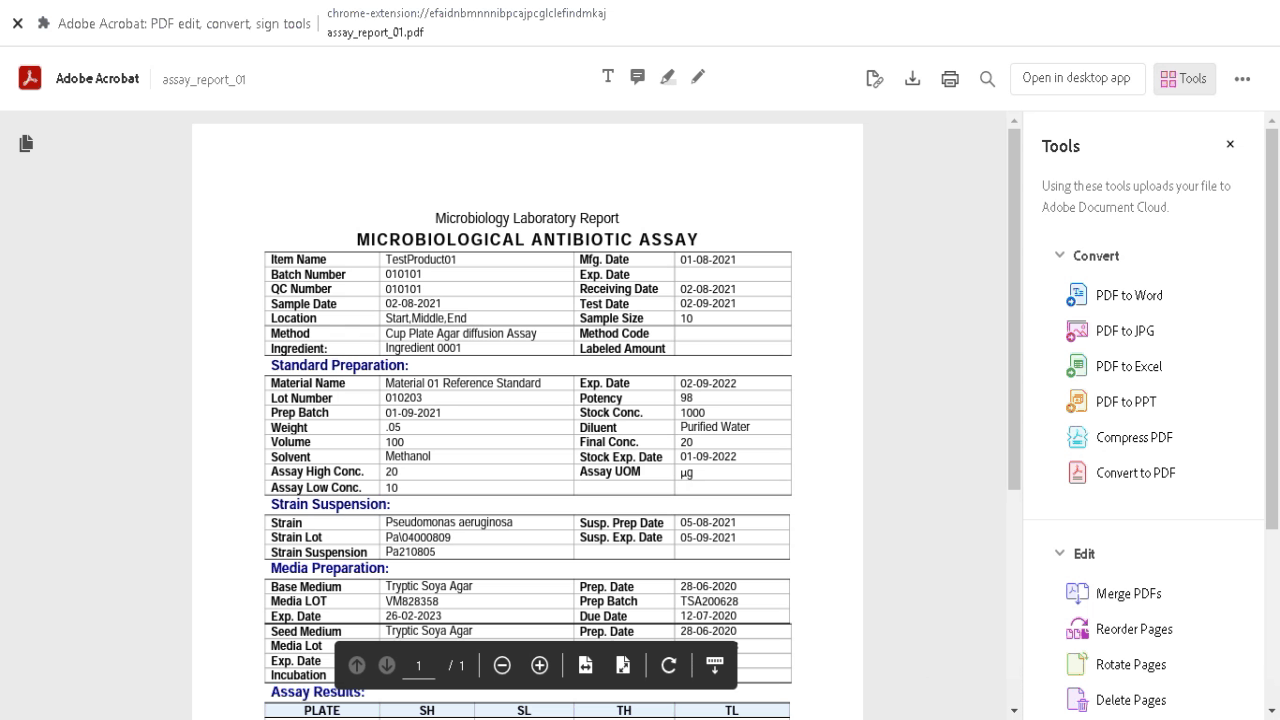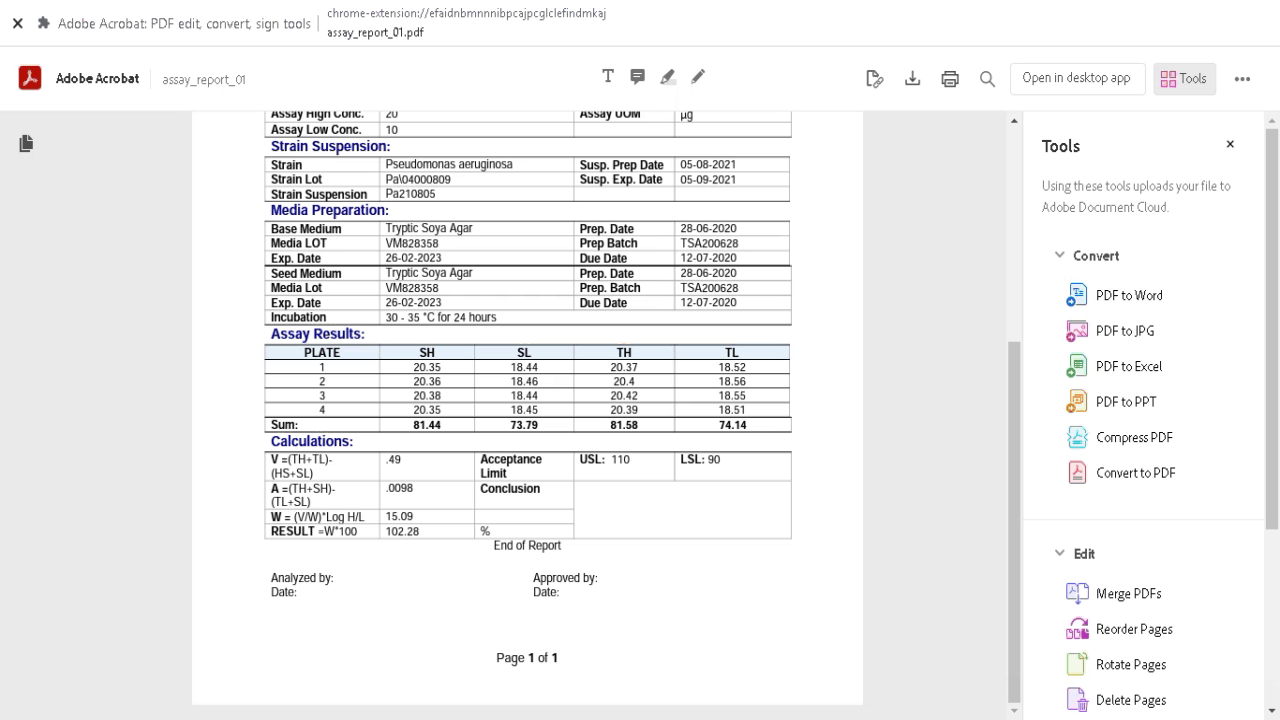
Demonstration of equivalent amounts of the same active pharmaceutical ingredient (API) between generic and innovator products (pharmaceutical equivalence) is a basic requirement of regulatory agencies for intravenous generic drugs prior to clinical use, and constitutes the pivotal point to assume therapeutic equivalence. Microbiological assay helps in estimating active constituents, biological activity and in monitoring the stability of antibiotics. Any small change in the antibiotic molecule, which may not be detected by chemical methods, will be revealed by a change in antimicrobial activity. Microbiological assay is very useful for resolving doubts regarding possible change in potency of antibiotics and their preparations.