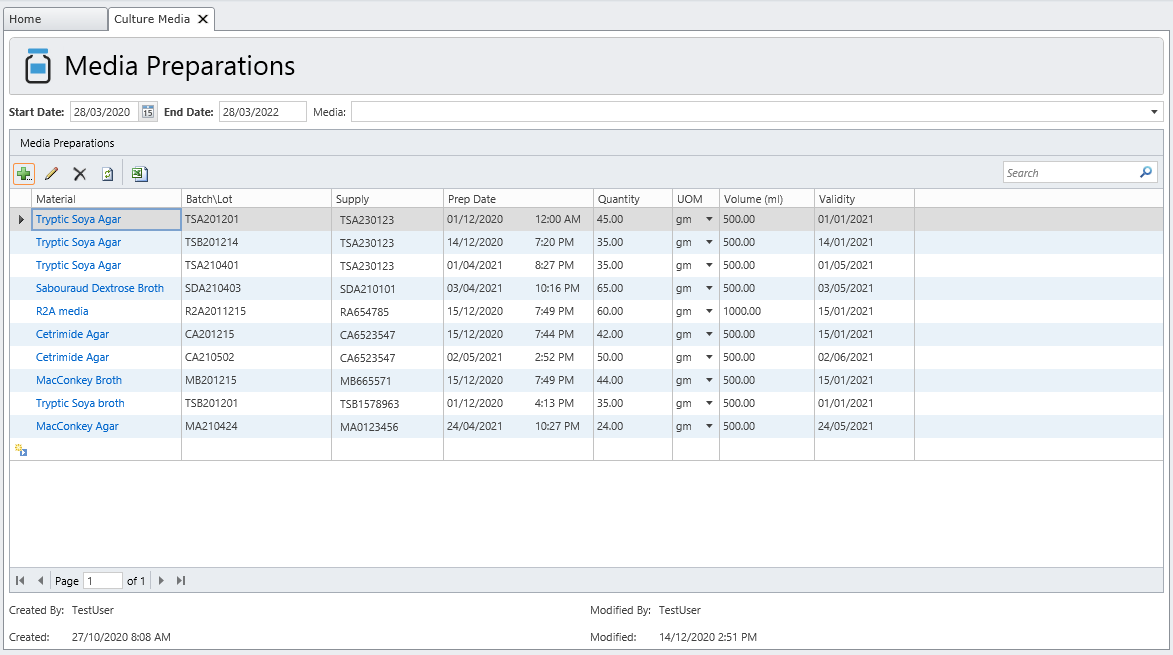
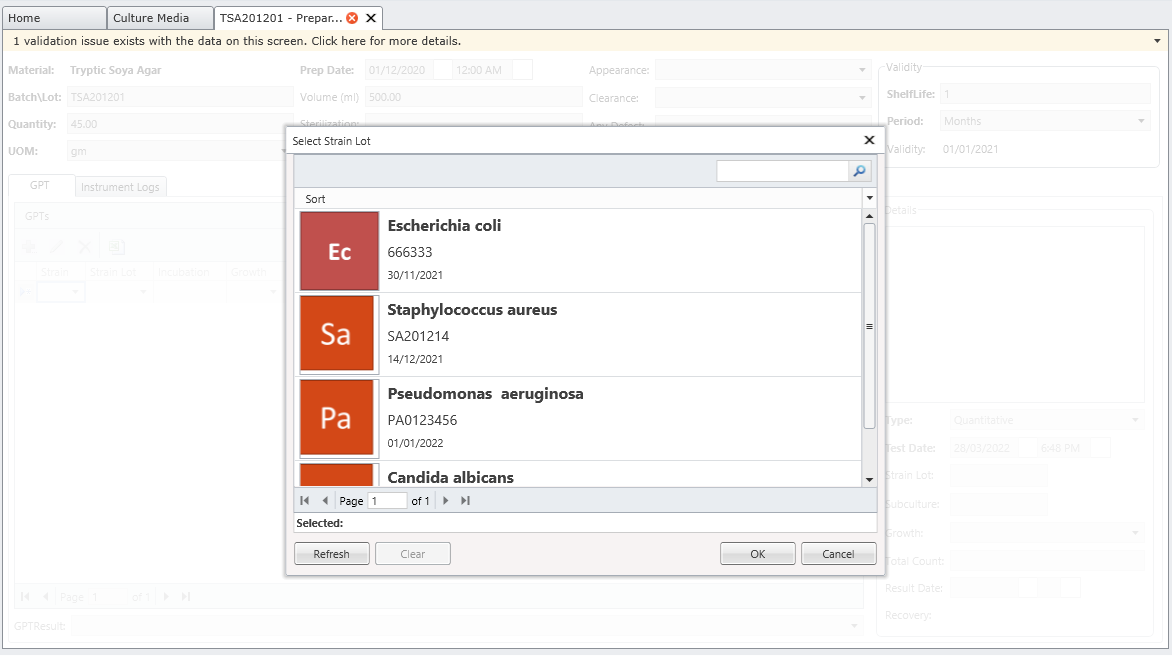
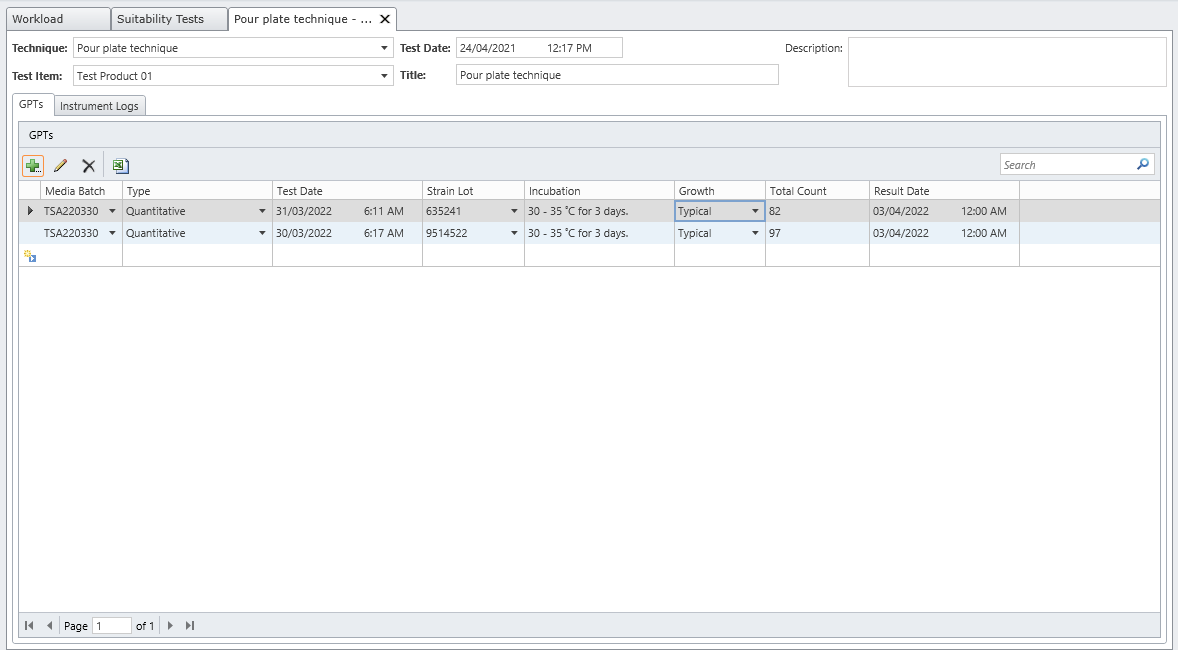
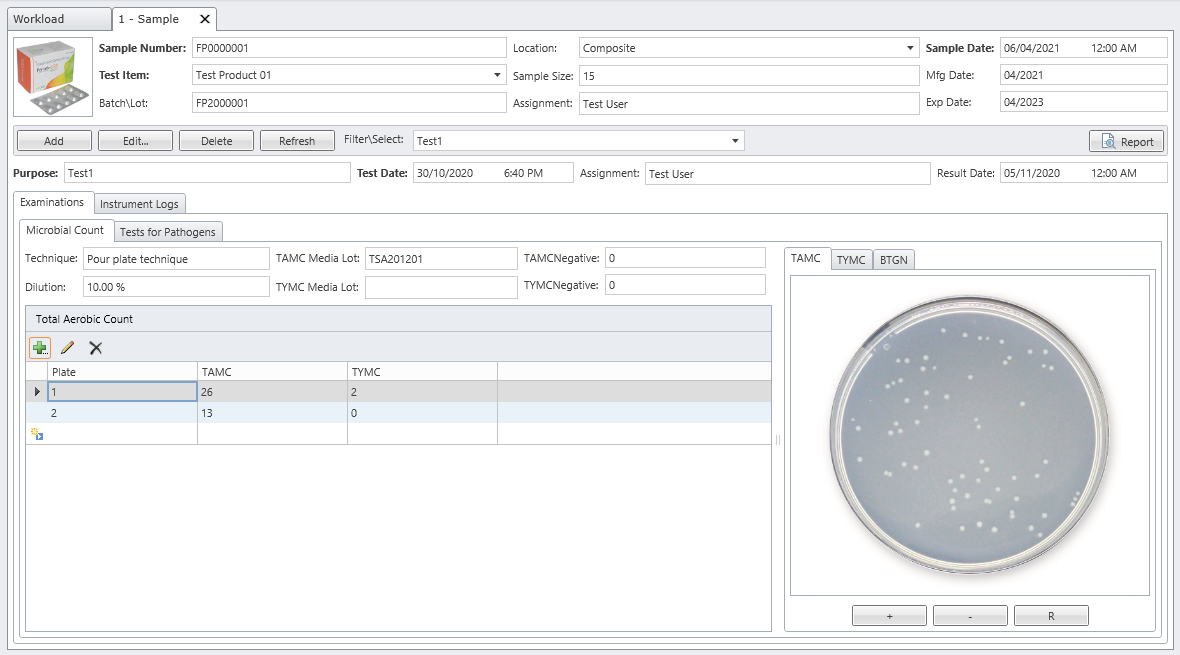
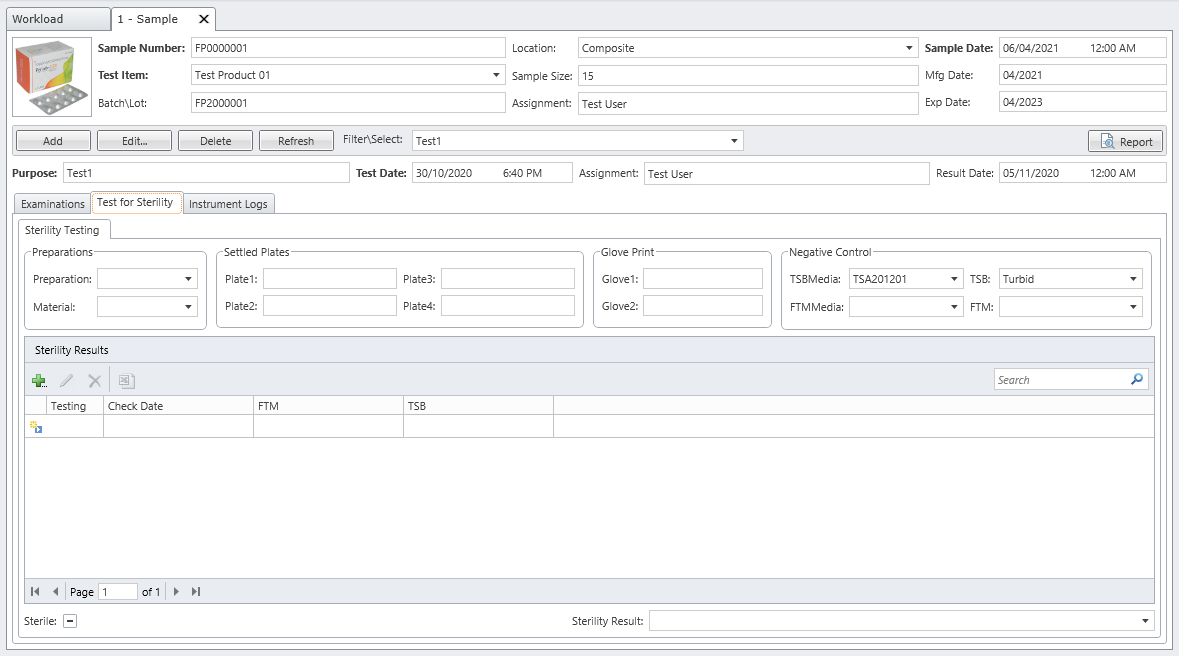
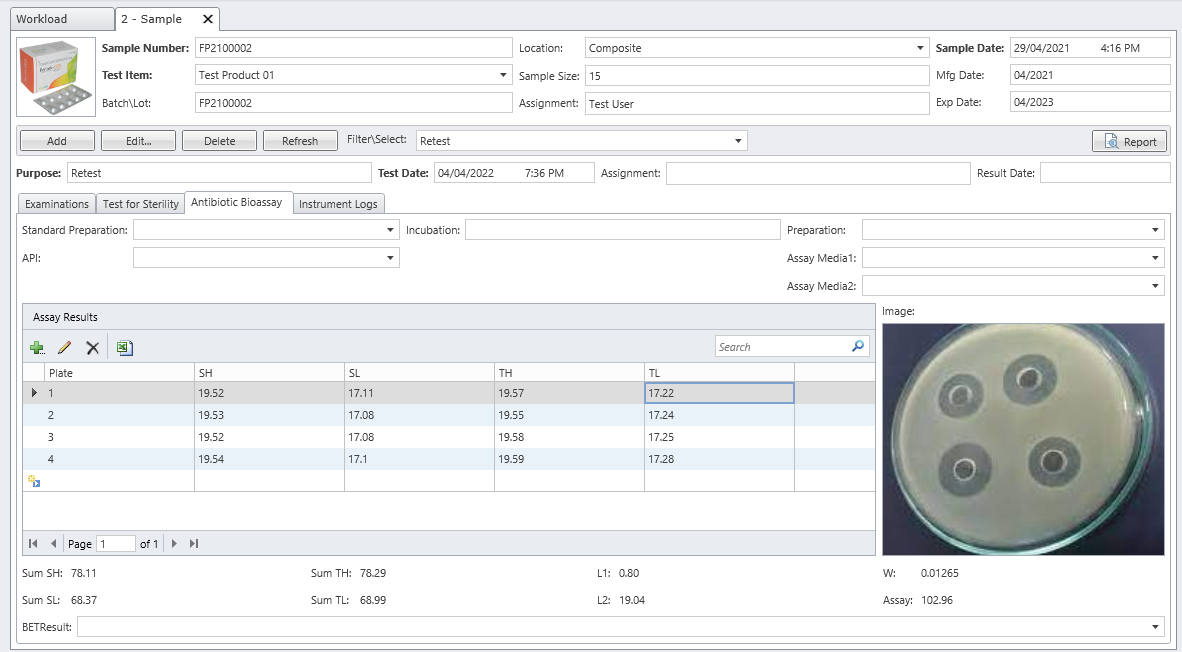
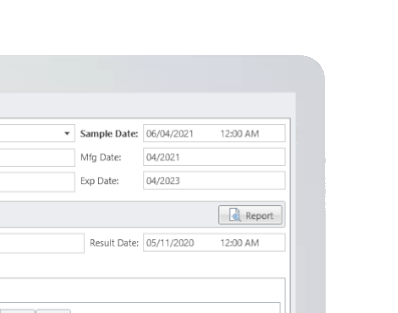
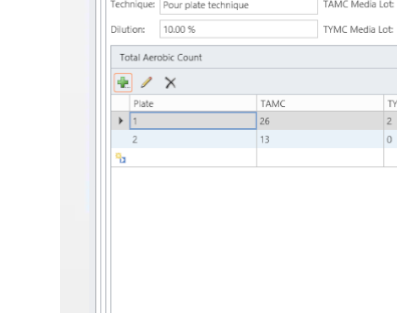
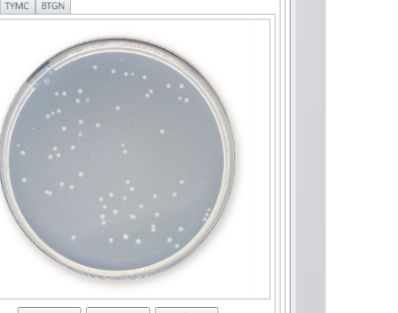
MicroLIMS is an innovative microbiology laboratory solution empowers microbiological examinations like test suitability, growth promotion tests (GPT), Microbiological Limits tests (MLT), Antibiotic bioassay, Sterility tests, Bacterial endotoxins, preservative and disinfectant efficacy test are included. Common laboratory activities like media preparations, reference strains and stock control in addition to assets and instrument logs. Control of samples that out of specifications (OOS) are conducted. Eliminate waste of time, Capture, organize, manage and collaborate test execution workflows across your organization with ease. With features designed to increase productivity while reducing errors. MicroLIMS lets you manage the sample life-cycle, optimize laboratory execution, perform data retrieval, interface instruments and systems, and enable security and auditing.
 |
 |
 |
 |
 |
 |
 |
 |
 |