Data Integration Services
Optimize your lab with seamless instrument integration, capturing data automatically for improved accuracy and efficiency.
Automated Data Capture
Optimizing your current investments in assets and planning for the future can be challenging without the right information. With integration solutions, instrument utilization information is automatically captured from both PC-based and non-PC-based instruments, regardless of manufacturer. Seamlessly integrating this information with service history enables more informed decisions relating to your capital equipment planning process and operating expenses.

The Challenge
Eliminating manual data entry significantly increases lab productivity and data integrity.
Unfortunately, instruments vary in how data is obtained. Optimal interface depends on compliance requirements, instrument type, and integration outcomes. Interface selection criteria involve security, integration, setup, and data extraction. The goal is maintain seamless connection between lab instruments and Silver-LIMS.
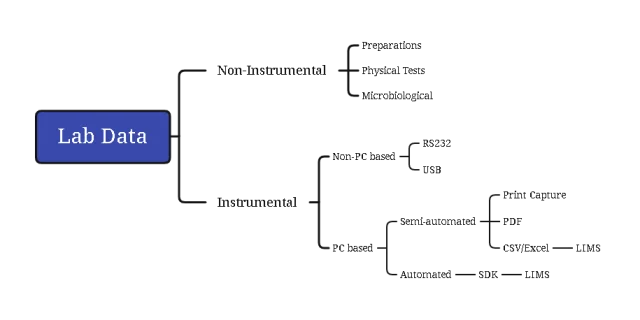
Our Solution
We offer two primary implementation methods based on customer requirements:
- Real-time Interface: Direct serial (RS-232) or network connection for immediate data capture. Ensures implicit data authenticity as manual steps are eliminated. Setup varies by vendor/model.
- File Share: Captures instrument report output via custom printer driver to a secure network file share. Handles all formats (Word, Excel, PDF). Requires robust access controls and backup processes.
Scientific Data Management System (SDMS)
The large volume of data generated by modern labs, along with regulatory requirements, forces companies to use LIMS efficiently. However, general-purpose LIMS often lack direct interfaces for all instruments. An SDMS provides "Print-to-Database" technology, facilitating the automatic entry of instrument reports directly into the database as Windows Enhanced Metafiles, minimizing data entry errors.

Instrument Interfacing & Compliance
FDA 21 CFR Part 11 Compliance:
Part 11 mandates accurate, reliable, and secure electronic records and signatures.
Validating your computer system is the primary means of ensuring compliance. Our platform
ensures that electronic records from instruments meet these rigorous standards.
File Attachment & Evidence
Some simple instruments (balances, pH meters) generate printed paper records. These original records must be signed, dated, and attached to batch records. CORPEX platform supports attachment of all evidential documents—analysis requests, instrument printouts, images—to ensure full traceability and data integrity.

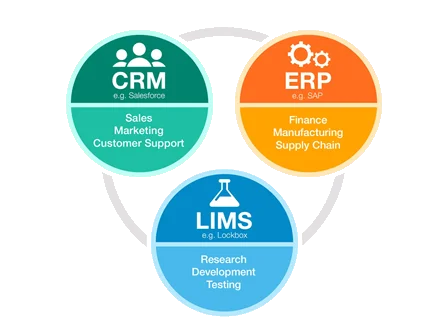
Integration with Other Firmware
Modern organizations rely on specialized software for different functions: CRM for sales/support, ERP for finance/manufacturing, and LIMS for R&D. Seamless integration between these systems (LIMS-ERP-CRM) is critical for holistic business management and data flow across departments, from research to production and sales.
Ready to Integrate Your Lab Data?
Contact us today to discuss your instrument interfacing and data integration needs.
Contact Us