Research and Development
Description
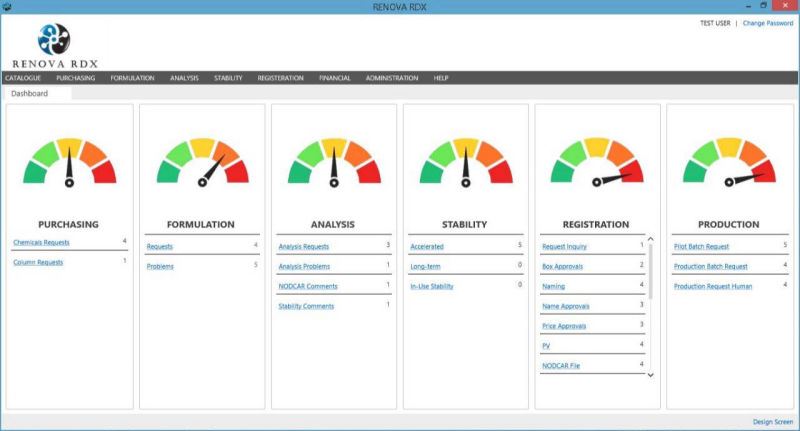
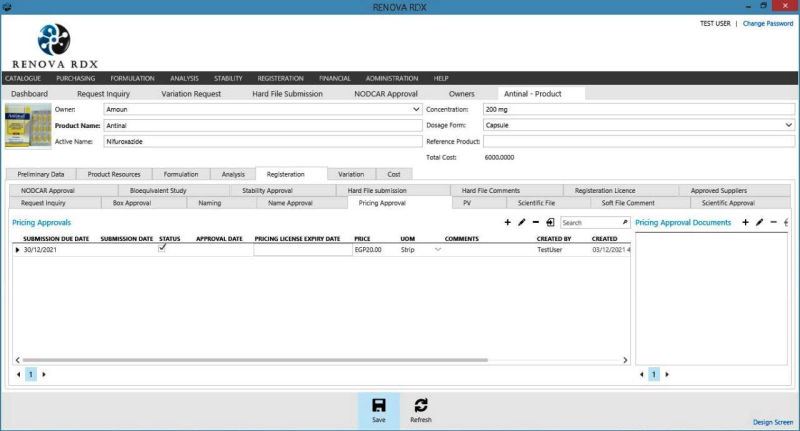
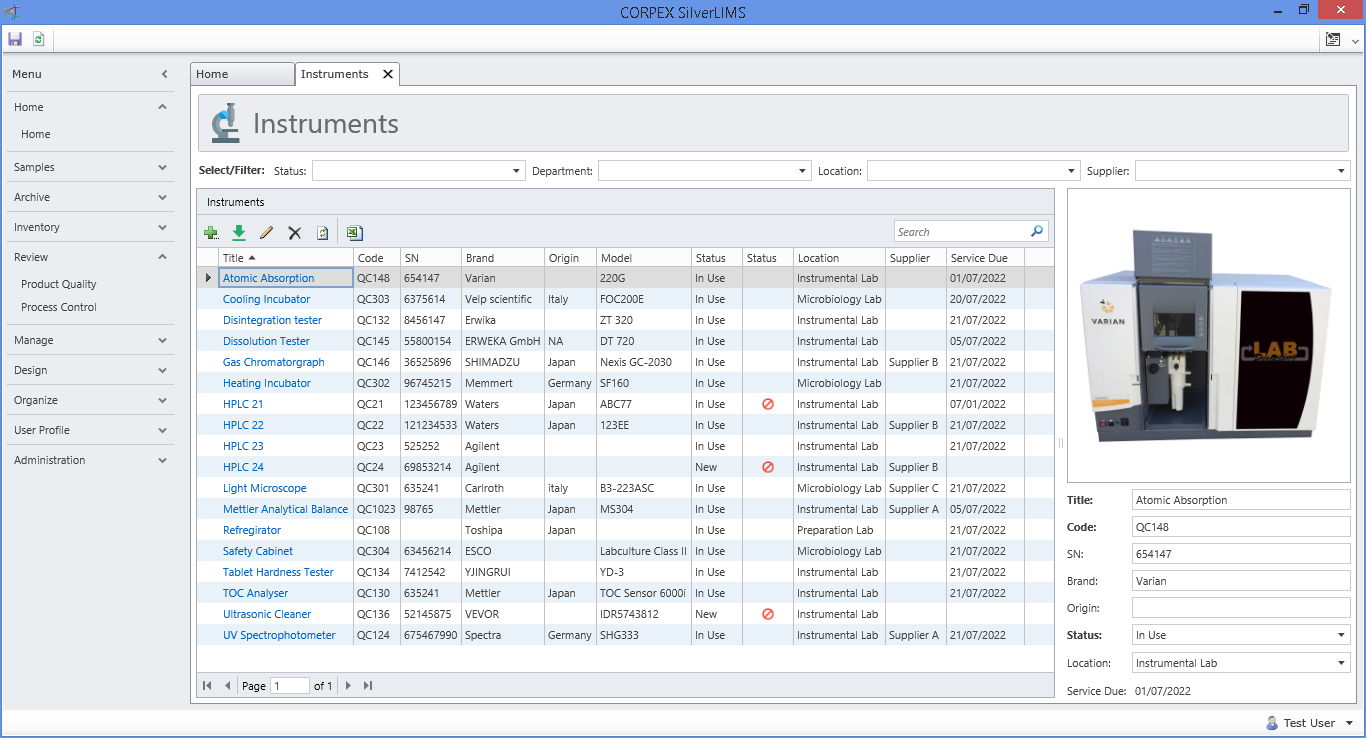
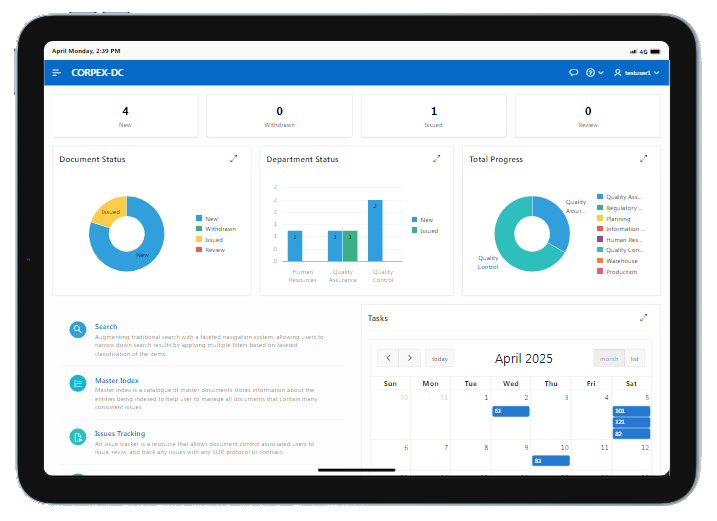
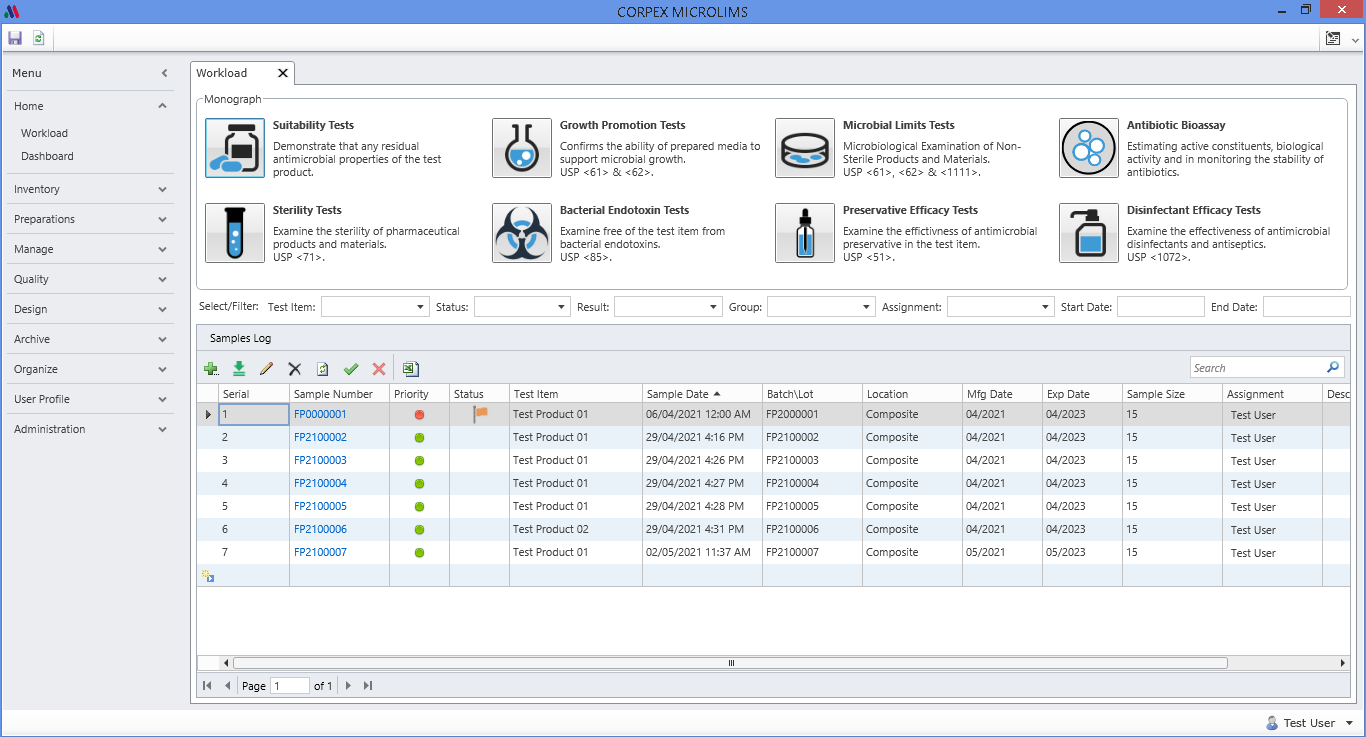
RENOVA RDX is a Modern integrated platform for pharmaceutical R&D and Regulatory affair. Renova RDX helps manufacturers maximize operational efficiency while assuring compliance with both internal corporate and external regulatory standards. It is a fully scalable system designed for organizations of any size. Task management gives users throughout the facility or organization appropriate access to input and retrieve data from a single, centralized system.