Leeuwenbook™
Portable Microbiology Laboratory Solution.
Mobile Lab Efficiency
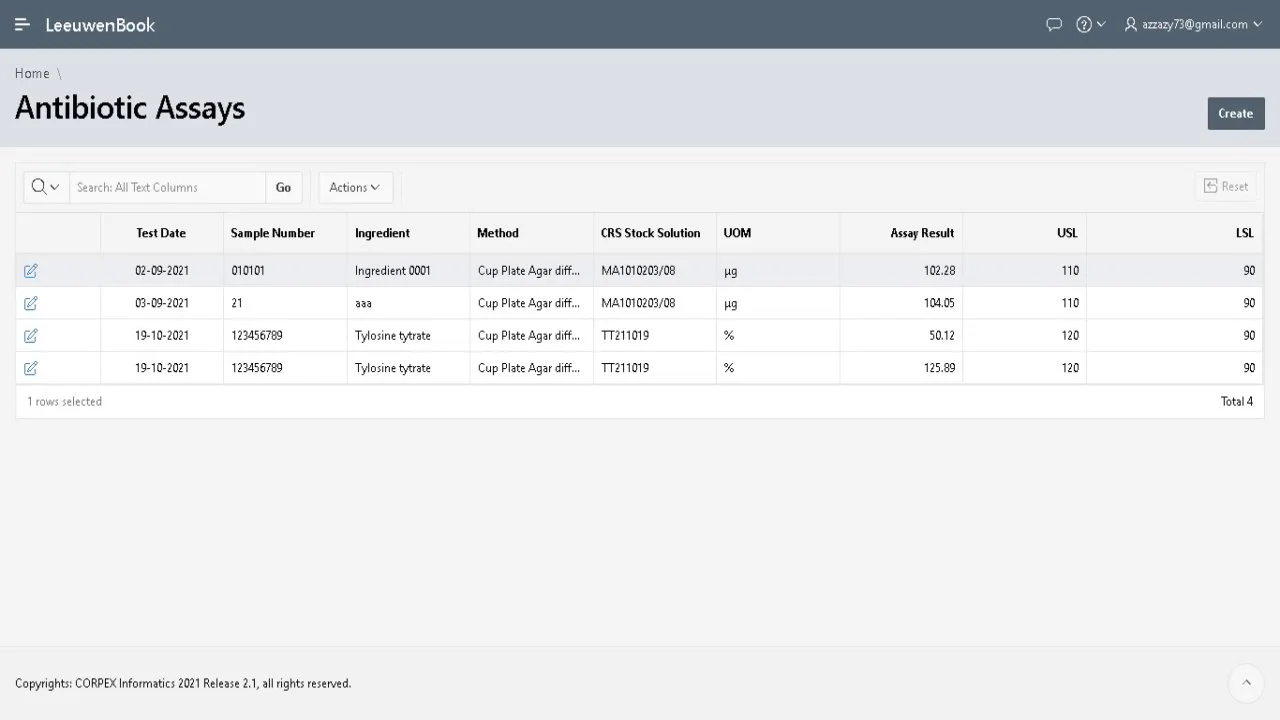
Leeuwenbook is a cutting-edge web and mobile application designed to streamline microbiology lab management. Named after Antonie van Leeuwenhoek, the "Father of Microbiology", this tool brings the power of a full LIMS to your portable devices.
Track sample status, manage tests, and generate reports on the go. Leeuwenbook covers a wide range of microbiological examinations including GPT, MLT, Bioassay, and Sterility tests, ensuring compliance and efficiency wherever you are.
Portable LIMS Benefits
Revolutionizing microbiological testing with mobility.
Mobility & Accessibility
Carry essential testing tools anywhere. Enables testing in remote fields, disaster response units for on-the-spot diagnostics.
Rapid Response
Facilitates point-of-care testing for quick turnaround times, critical for effective disease control and patient management.
Real-time Data
Capture, analyze, and share data instantly across locations. Enhances collaboration among researchers and public health authorities.
Enhanced Efficiency
Streamlines workflows and reduces manual errors through automation and electronic data capture.
Cost-effectiveness
Reduces need for extensive infrastructure at every location. Saves on transportation and resource utilization.
EHR Integration
Seamlessly integrates with Electronic Health Records to ensure test results are readily available to healthcare providers.
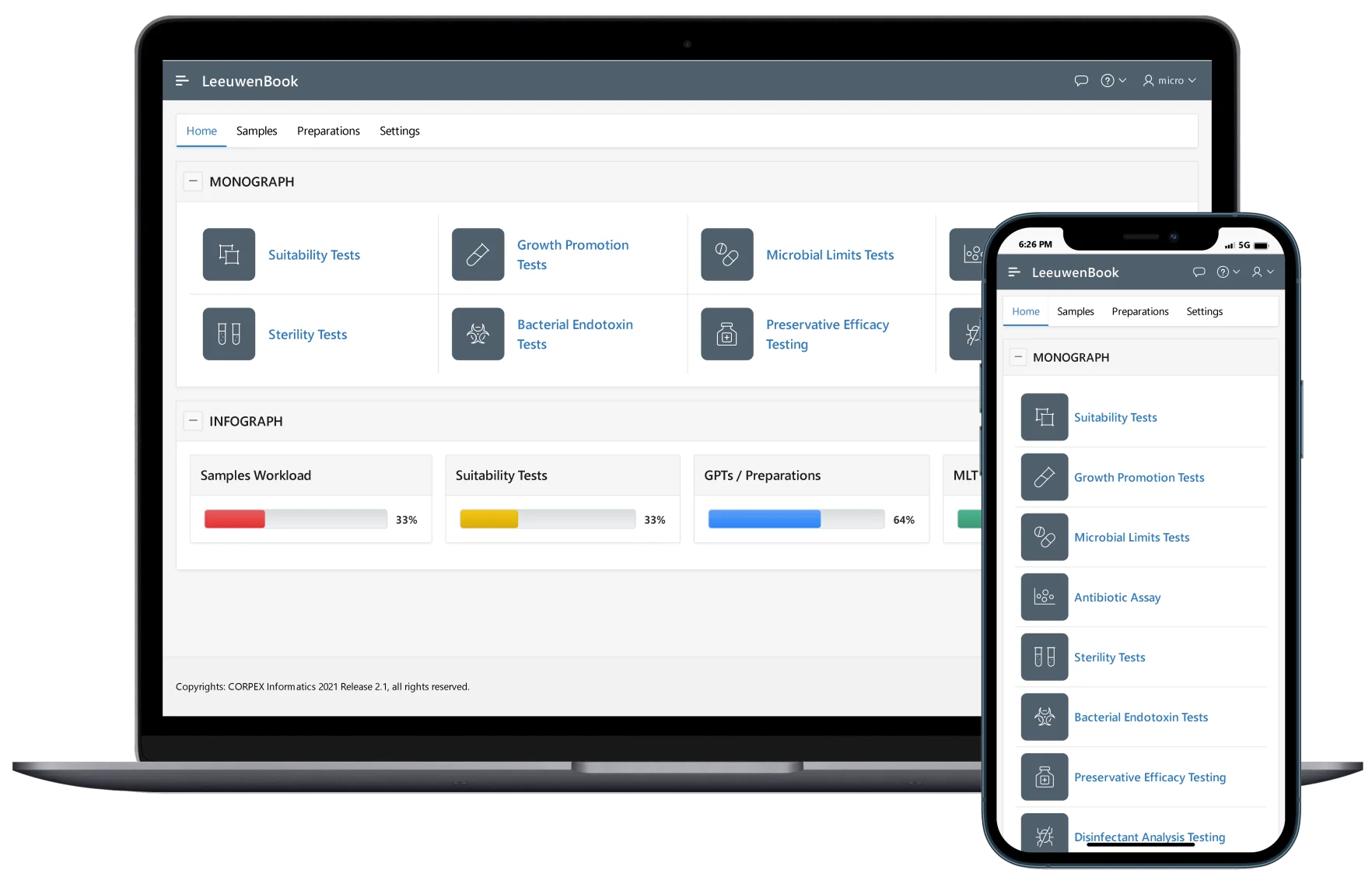
Media Preparation & GPT
Microbial culture media preparation is routine but critical. Leeuwenbook provides recording of all pertaining information for culture media, subculture of Certified Reference Microorganisms (CRMs), and integrates laboratory preparation workflows.
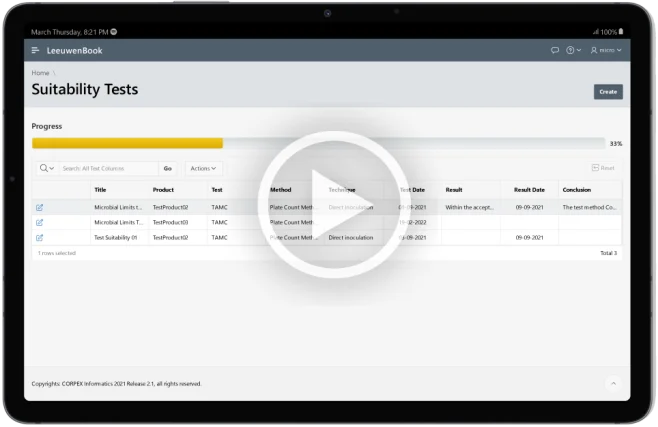
Suitability Tests
Demonstrate that any residual antimicrobial properties have been neutralized. Leeuwenbook integrates pertaining information of various suitability methods, ensuring compliance with USP standards.
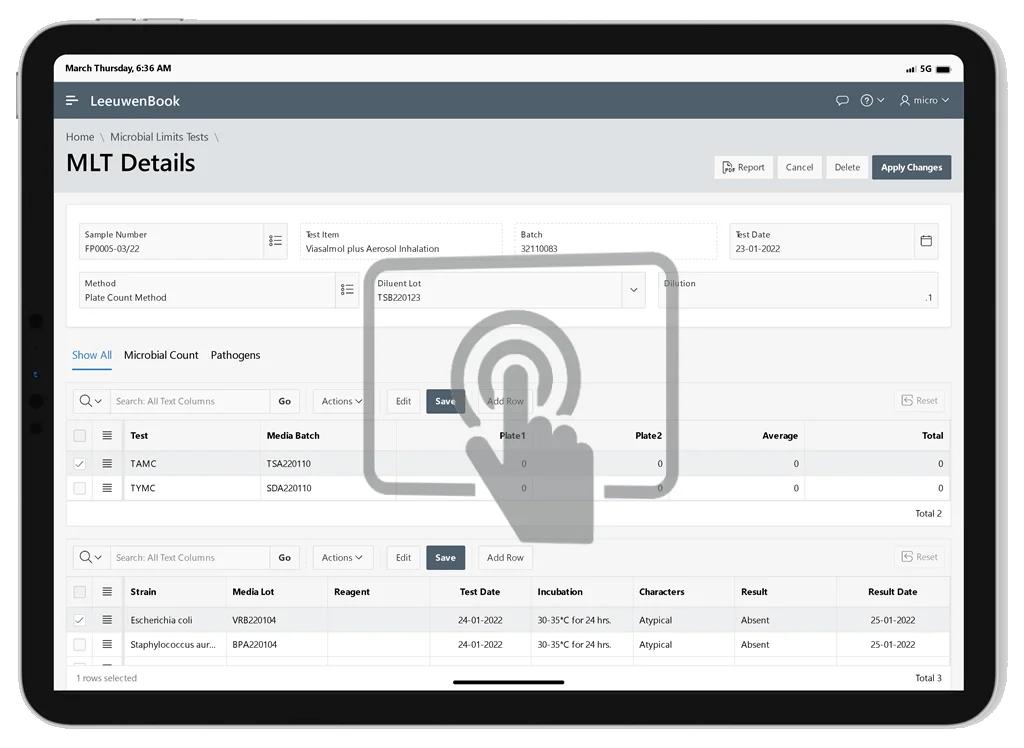
Microbial Limits Tests (MLT)
Assess viable microorganisms in non-sterile products. Leeuwenbook helps determine whether a substance complies with established specifications for microbiological quality.
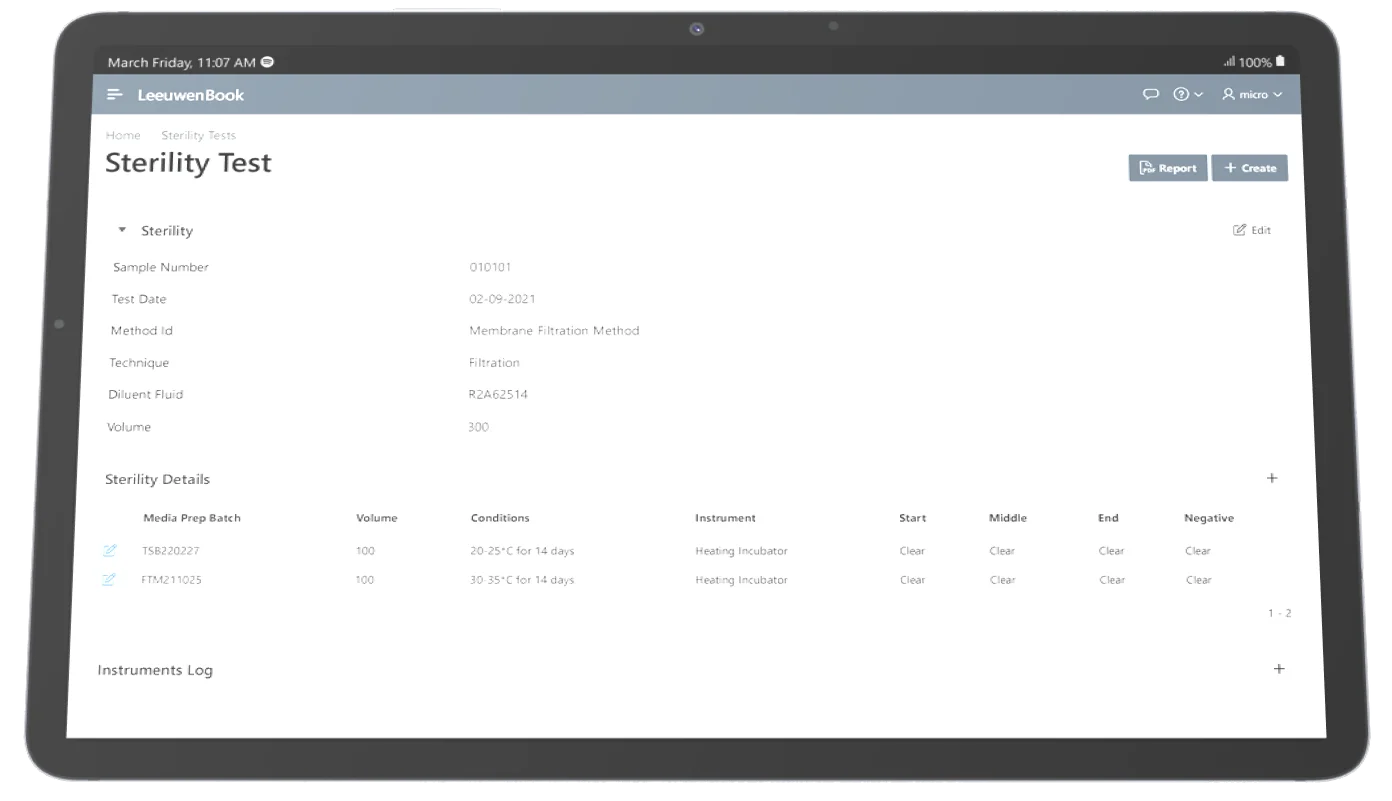
Sterility Tests
Confirm sterile products do not contain viable microorganisms. Leeuwenbook supports accurate sterility testing methods (membrane filtration or direct inoculation) crucial for medical devices and pharma products.
Go Mobile with Leeuwenbook
Experience the freedom of a portable microbiology lab management system.
Contact Us