StabLIMS™
Innovative Solution for Pharmaceutical Stability Laboratories.
Stability Laboratory Information Solution
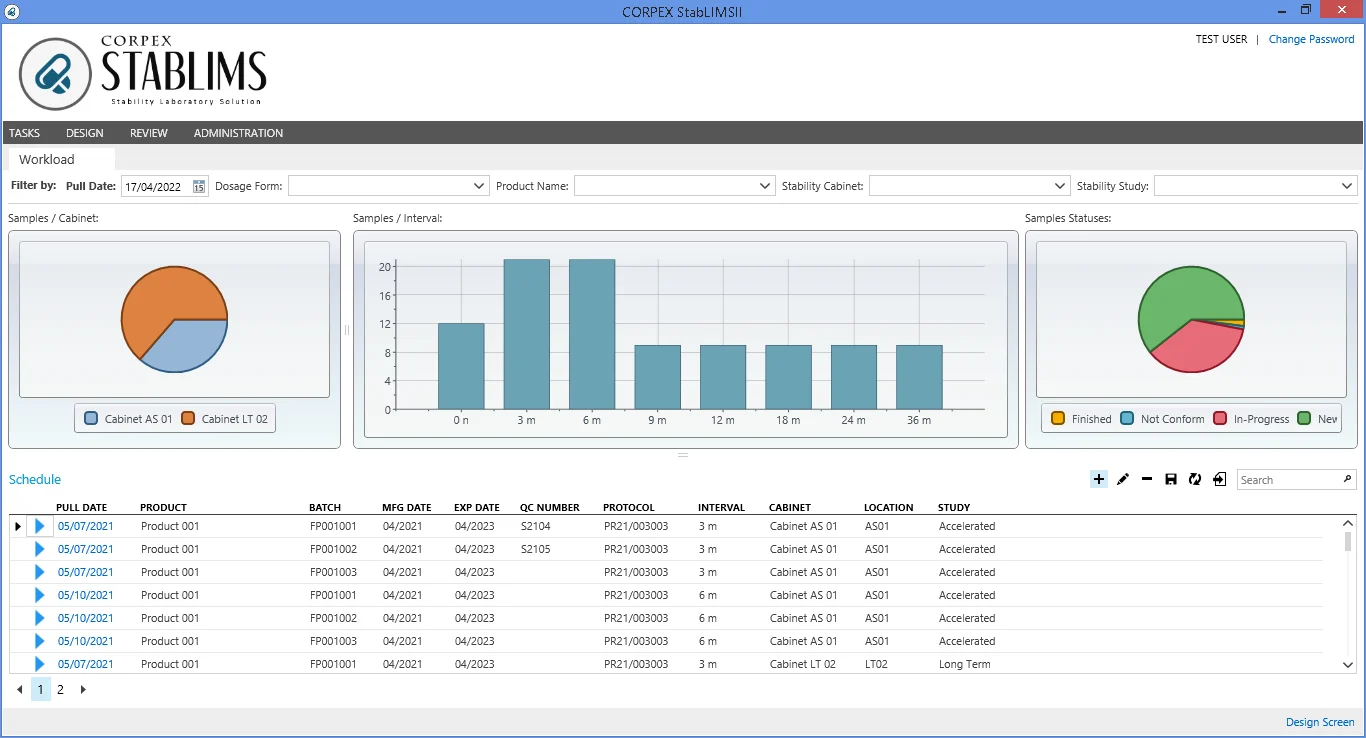
STABLIMS™ supports all types of stability studies which includes most recognized climatic zones. Incubation temperature and relative humidity are the essential requirements of most studies; moreover frequency of testing and acceptance pulling days should be determined to automate the workload.
Determination testing intervals and infrastructure of inventory design including sample location are key features of the system.
Upgrade Your Lab with Elegant Solutions
STABLIMS™ aims to manage the day to day activities of stability department either in quality, research and development boards. The integrated system complies with current medicinal product regulations; FDA, WHO, CDER, ICH, ISO and CFR 21 Part 11 to manage and automate all types of drug products.
STABLIMS™ offers electronic worksheets that result in faster data entry, improve efficiency and reduce manual error. You have the advantage of complete tracking without ever having to print out anything. Our software meets up to 95% of customer requirements while still allowing the remaining 5% to be tailored to fulfill unique needs.
Ready to Automate your Stability Studies?
Contact us today to learn more about how StabLIMS can streamline your operations.
Contact Us